Home» Pharmaceutical Analysis» Unknown Peak Identification
CLL Workflow for Unknown Peak Identification
Identification of unknown peaks in is now a regulatory requirement. Any unknown peak(s) eluting in LC chromatograms are required to be identified and reviewed to check whether it is API, Excipient or Process related. CLL’s LC-QTOF workflow can make unknown peak identification a breeze.

Why is unknown impurity identification useful?
- OOS Investigations
- Stability Samples
- Forced Degradation
- Related Substances analysis
- New molecule development
- Cleaning Validation
- Formulation development – Impurity profiling
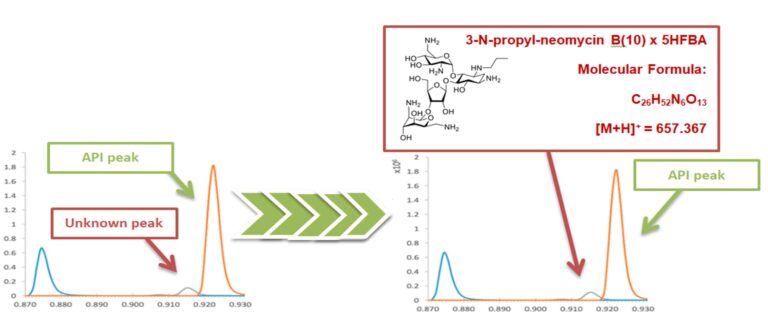
Unknown Peak Identification Graph
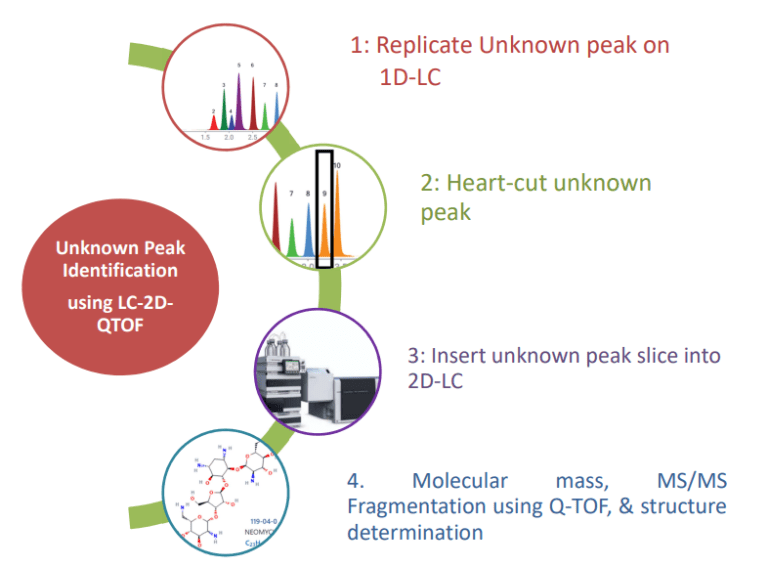
Advantages of CLL Workflow
- No MS compatible method
development needed. - CLL will replicate your method on LC using your own sample preparations, mobile phase and column.
- Saves investigation &
laboratory time
Information from the CLL’s Standard Unknown Impurity Report?
- Molecular Chemical Formula
- Molecular Mass
- MS and MS2 fragments
- Probable identification with Structure and stated mass accuracy
Additional Information from CLL’s Advanced Report
- Product or process related impurity
- Potential source of impurity determined – placebo, reagents, process carry-over, etc.
Sample Volume Required:
Prepared Blank, Standard and Sample vials (1mL each), Mobile phase (1 – 2 Liters), Placebo (5grams), API (2grams).
Additional information required at time of submission for exact
replication of your method and unknown impurity – Column, Your Chromatogram with unknown peak and API peak marked, Instrument
method, STP and Specification.
Turnaround time: 5 working days; Express 48 hoursservices available.